Tungsten Carbide - Hard Metal
PRODUCTS AND TECHNICAL CONSULTING TO CUSTOMER SERVICE
Want to be sure of the product to buy?

What is Tungsten Carbide?
Tungsten carbide (hard metal)
Cemented carbides, or hardmetals as they are often called, are materials made by "cementing" very hard tungsten monocarbide (WC) grains in a binder matrix of a tough cobalt or nickel alloy by liquid phase sintering. Cemented carbides combine the high hardness and strength of metallic carbides (WC, TiC, TaC) or carbonitrides (eg TiCN) with the toughness and plasticity of a metallic alloy binder (Co, Ni, Fe), in which the hard particles are evenly distributed to form a metallic composite. Tungsten carbide is the most metallic of the carbides, and by far the most important hard phase. The more hard carbide particles are within the material, the harder it is but the less tough it behaves during loading; and, vice versa, significant increases in toughness are achieved by a higher amount of metallic binder at the expense of hardness.
Within the field of engineering materials, cemented carbides play a crucial role as they combine high hardness and strength with good toughness within a wide property range, and thus constitute the most versatile hard materials group for engineering and tooling applications.
Typically a Tungsten Carbide Hard Metal can have a hardness value of 1600 HV, whereas mild steel would be in the region of 160 HV a factor of 10 lower.
Although called a hard metal, Tungsten Carbide is actually a composite material with hard particles of Tungsten Carbide embedded in a softer matrix of metallic Cobalt .
The chemical formula for Tungsten Carbide is WC.
Tungsten carbide is prepared by reaction of tungsten metal and carbon at 1400–2000 °C. Other methods include a patented lower temperature fluid bed process that reacts either tungsten metal or blue WO3 with CO/CO2mixture and H2 between 900 and 1200 °C.
What are the properties of hard metal?
Hard metal properties can be summarized as follows:
Mechanical properties:
Mechanical properties reflect the ability of a material to withstand a force of a certain type that, however, when applied, requires a more accurate definition. For clarity there are various types of force to which the resistance is opposed, depending on the load conditions; for hard metals the most important resistances within mechanical properties are compression resistance
traction, abrasion, fatigue and bump transversal breakage .:
Physical properties:
Tungsten carbide has a high melting point at 2,870 °C (5,200 °F), a boiling point of 6,000 °C (10,830 °F) when under a pressure equivalent to 1 standard atmosphere (100 kPa), a thermal conductivity of 110 W·m−1·K−1, and a coefficient of thermal expansion of 5.5 µm·m−1·K−1.
Tungsten carbide is extremely hard, ranking about 9 on Mohs scale, and with a Vickers number of around 2600. It has a Young's modulus of approximately 530–700 GPa, a bulk modulus of 630–655 GPa, and a shear modulus of 274 GPa. It has an ultimate tensile strength of 344 MPa, an ultimate compression strength of about 2.7 GPa and a Poisson's ratio of 0.31.
The speed of a longitudinal wave (the speed of sound) through a thin rod of tungsten carbide is 6220 m/s.
Tungsten carbide's low electrical resistivity of about 0.2 µΩ·m is comparable with that of some metals (e.g. vanadium 0.2 µΩ·m).
WC is readily wetted by both molten nickel and cobalt. Investigation of the phase diagram of the W-C-Co system shows that WC and Co form a pseudo binary eutectic. The phase diagram also shows that there are so-called η-carbides with composition (W,Co) 6C that can be formed and the brittleness of these phases makes control of the carbon content in WC-Co hard metals important.
Chemical properties:
There are two well-characterized compounds of tungsten and carbon, WC and tungsten semicarbide, W
2C. Both compounds may be present in coatings and the proportions can depend on the coating method.
At high temperatures WC decomposes to tungsten and carbon and this can occur during high-temperature thermal spray, e.g., in high velocity oxygen fuel (HVOF) and high energy plasma (HEP) methods.
Oxidation of WC starts at 500–600 °C (932–1,112 °F). It is resistant to acids and is only attacked by hydrofluoric acid/nitric acid (HF/HNO3) mixtures above room temperature.
It reacts with fluorine gas at room temperature and chlorine above 400 °C (752 °F) and is unreactive to dry H2 up to its melting point. Finely powdered WC oxidizes readily in hydrogen peroxide aqueous solutions. At high temperatures and pressures it reacts with aqueous sodium carbonate forming sodium tungstate, a procedure used for recovery of scrap cemented carbide.
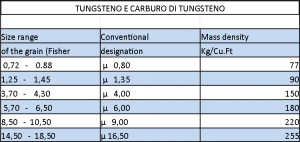
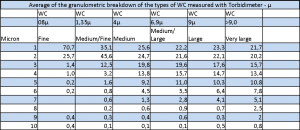
Hard metal grain size tables: